When we talk about obesity, most people focus on excess fat. But a closer look at the science—and the figure below—reveals a deeper story: obesity is a disorder of ECS dysfunction, with dramatic, organ-specific changes that drive disease far beyond simple weight gain.
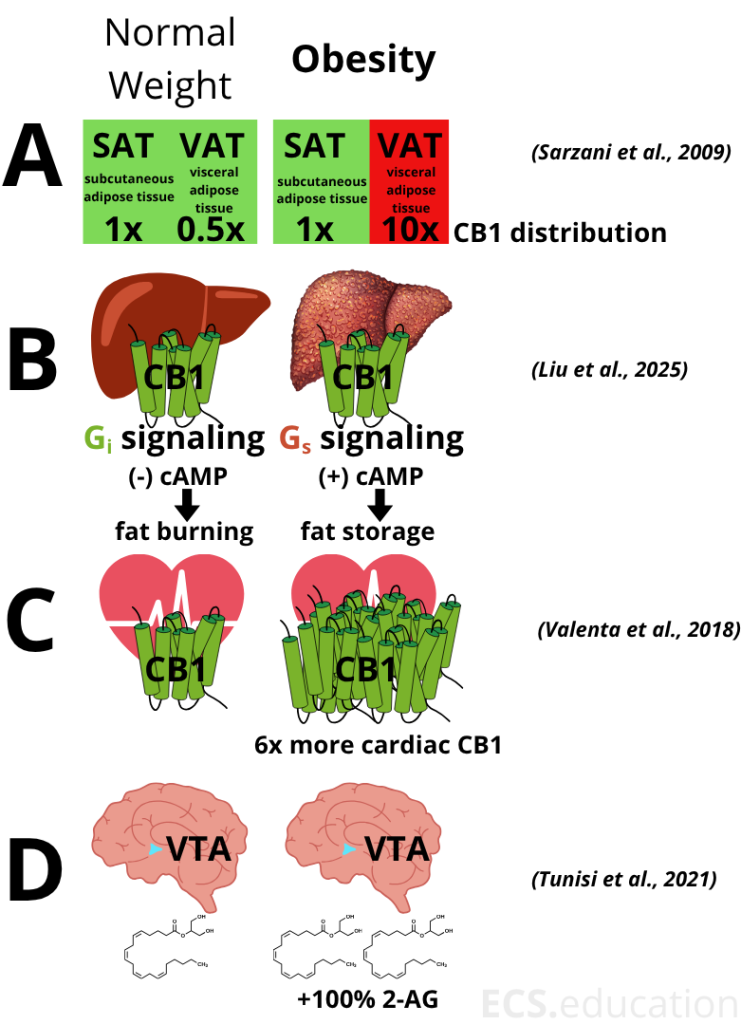
Panel A: Adipose Tissue – CB1 Moves to the Danger Zone
In healthy, normal-weight individuals, CB1 cannabinoid receptors are more abundant in subcutaneous fat (SAT, just under the skin) than in visceral fat (VAT, around the organs). But obesity flips this pattern: CB1 expression in VAT skyrockets, becoming ten times higher than in normal-weight individuals, while SAT CB1 remains unchanged (Sarzani et al. 2009). This shift means VAT, the depot most linked to insulin resistance and inflammation, becomes a hotspot for ECS overactivity.
Why Does This Matter? CB1 in Visceral Fat Drives Disease
High CB1 in VAT is tightly linked to increased waist circumference, insulin resistance, and metabolic syndrome. The ECS in VAT becomes a driver of chronic disease, not just a bystander.
Panel B: The Liver – CB1 Signaling Flips from Fat Burning to Fat Storage
The liver tells a similar story of ECS rewiring. In normal-weight individuals, hepatic CB1 activation promotes fat burning (via Gi protein signaling and reduced cAMP). But in obesity, the same receptor switches to Gs protein signaling, increasing cAMP and promoting fat storage (lipogenesis). Recent work by Liu et al. (2025) demonstrates this “signaling switch,” which helps explain why fatty liver disease is so common in obesity and why CB1 antagonists can reverse it.
Panel C: The Heart – Cardiac CB1 Upregulation and Cardiometabolic Risk
Obesity doesn’t stop at fat and liver. As shown by Valenta et al. (2018), cardiac CB1 receptors are massively upregulated in obesity (~+600%), both in animal models and humans. This upregulation is associated with increased local endocannabinoid levels and is implicated in cardiac dysfunction, inflammation, and possibly the development of obesity-related cardiomyopathy.
Panel D: The Brain – ECS Overactivity and Hedonic Drive
The ECS’s influence extends to the brain and our reward system. Hypothalamic CB1 receptors regulate energy balance and food intake. Obesity is associated with increased hypothalamic endocannabinoid tone and a doubling of 2-AG in the ventral tegmental area (VTA), driving hedonic eating and hyperphagia (Tunisi et al., 2021). This chronic CB1 overactivity in reward circuits further compounds metabolic dysfunction and addictive food behaviors.
Why Does This Happen? The Precursor-Driven System
The ECS is tightly linked to dietary fats and metabolic state. High intake of omega-6 fatty acids (like linoleic acid) and chronic inflammation drive up endocannabinoid production, which in turn upregulates CB1 in visceral fat, liver, and heart. This creates a vicious cycle: more CB1 means more fat storage, more inflammation, and more metabolic disease.
Key Takeaway: Obesity Is an ECS Dysfunction Disorder
Obesity doesn’t just add fat. It fundamentally rewires your endocannabinoid system. This rewiring locks in metabolic dysfunction, driving insulin resistance, fatty liver, heart disease, addictive food behaviours, and chronic inflammation. Understanding and targeting these ECS changes offers a powerful new avenue for prevention and treatment.
Bottom Line:
Obesity is not just about excess calories or fat—it is a state of ECS dysregulation that touches every major metabolic organ. Restoring ECS balance could be the key to reversing the tide of metabolic disease.
References:
Sarzani R, Bordicchia M, Marcucci P, et al. Altered pattern of cannabinoid type 1 receptor expression in adipose tissue of dysmetabolic and overweight patients. Metabolism. 2009;58(3):361-367. doi:10.1016/j.metabol.2008.10.009
Liu J, Oliverio A, Godlewski G, et al. Hepatic CB1 receptor signaling triggers Gi/oα-mediated lipolysis in lean mice but Gsα-mediated lipogenesis in obese mice. Metabolism. Published online May 28, 2025. doi:10.1016/j.metabol.2025.156308
Valenta I, Varga ZV, Valentine H, et al. Feasibility Evaluation of Myocardial Cannabinoid Type 1 Receptor Imaging in Obesity: A Translational Approach. JACC Cardiovasc Imaging. 2018;11(2 Pt 2):320-332. doi:10.1016/j.jcmg.2017.11.019
Tunisi L, D’Angelo L, Fernández-Rilo AC, et al. Orexin-A/Hypocretin-1 Controls the VTA-NAc Mesolimbic Pathway via Endocannabinoid-Mediated Disinhibition of Dopaminergic Neurons in Obese Mice. Front Synaptic Neurosci. 2021;13:622405. Published 2021 Feb 4. doi:10.3389/fnsyn.2021.622405


Comment
Comments are closed.