When scientific tunnel vision becomes institutional blindness
Lessons from history’s scientific blind spots
Science has a troubling habit of missing the forest for the trees. For decades, gastroenterologists dismissed the idea that bacteria could cause stomach ulcers—until Barry Marshall proved Helicobacter pylori was the culprit by infecting himself. Geologists ridiculed continental drift theory for fifty years before plate tectonics vindicated Wegener. Cardiologists focused obsessively on cholesterol while ignoring inflammation, until C-reactive protein revolutionized cardiac risk assessment.
The pattern is always the same: rigid specialization creates intellectual silos, and critical connections get lost in the gaps between disciplines.
Today, we’re witnessing another spectacular example of this phenomenon. One that may be costing lives through therapeutic tunnel vision.
The biochemical question that should unite two fields
Here’s a simple question that should have obvious implications: If arachidonic acid serves as the precursor for both eicosanoids and endocannabinoids, surely the inflammation and endocannabinoid research communities must collaborate extensively?
Both signaling systems:
- Derive from identical substrate (arachidonic acid)
- Operate in identical concentration ranges (picomolar to nanomolar)
- Regulate inflammation, pain, and tissue homeostasis
- Respond to identical dietary and pathological triggers
Logic suggests these fields would be intimately connected, with shared conferences, collaborative studies, and integrated therapeutic approaches.
The reality is far more sobering.
Case study: two studies, one phenomenon, zero cross-talk
Consider two landmark 2019 papers that should have been published as companion pieces:
Kuipers et al. meticulously documented how high-fat, omega-6-rich diets increase circulating endocannabinoids—2-AG rising 3.4-fold and AEA climbing 2.2-fold over 20 weeks. Their focus: how substrate availability drives endocannabinoid synthesis and metabolic dysfunction.
Declèves et al. simultaneously demonstrated that identical dietary interventions dramatically elevate arachidonic acid-derived eicosanoids—with some molecules like 8,9-EET surging an extraordinary 12.8-fold over 16 weeks. Their focus: how the same substrate feeds inflammatory cascade activation.
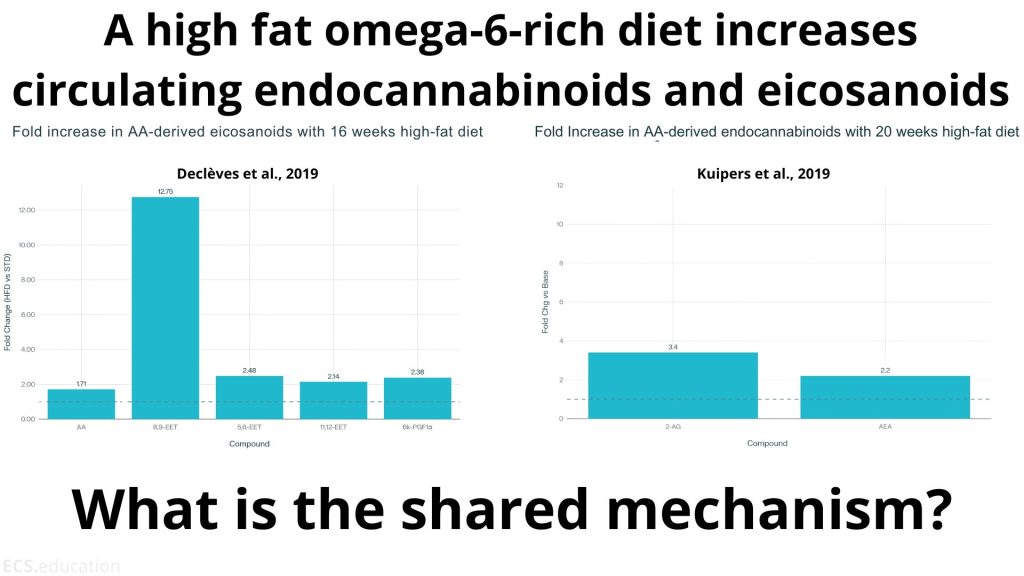
The absurdity? Both groups studied the exact same biochemical phenomenon while remaining hermetically sealed in their respective academic universes. Same dietary trigger. Same molecular substrate. Same temporal progression. Same tissue dysfunction. Two halves of one integrated response, published in isolation.
Neither paper acknowledges the parallel pathway. Kuipers discusses elevated arachidonic acid without mentioning eicosanoid synthesis. Declèves examines PLA2-mediated AA release while ignoring endocannabinoid production. It’s as if two teams of researchers studied different aspects of photosynthesis while pretending the other team didn’t exist.
The 2025 Reality Check: Still Blissfully Unaware
If this were simply a historical quirk, we might dismiss it as temporary growing pains. Unfortunately, a brand new 2025 review reveals that nothing has changed.
Kolawole and Kashfi just published “Reprogramming inflammation: Mechanisms and therapeutic targeting of eicosanoids and pro-resolving mediators”—a comprehensive treatise on arachidonic acid-derived inflammatory mediators. The authors meticulously catalog PLA2-mediated substrate release, enumerate enzymatic pathways, and discuss specialized pro-resolving mediators operating in “picomolar to nanomolar concentrations” for “immunomodulation, tissue repair, and barrier function restoration.”
Not once do they mention endocannabinoids.
Despite describing the identical substrate, identical concentration ranges, and identical therapeutic objectives, these inflammation specialists remain blissfully unaware that half the arachidonic acid story exists in a parallel research universe.
The paper’s keywords tell the tale: “Antiinflammatory; Cytochrome P450; Inflammation; Leukotrienes; Prostaglandins; Resolution; Resolvins; Specialized pro-resolving mediators.”
Absent: Any mention of endocannabinoids, cannabinoids, 2-AG, AEA, CB1, CB2, or the entire endocannabinoid system.
The cost of intellectual apartheid
This is not merely academic pedantry, no it has real consequences:
Therapeutically crippled strategies
Targeting eicosanoid pathways while ignoring endocannabinoid modulation is like conducting an orchestra with half the instruments silenced. The endocannabinoid system represents evolution’s own inflammation resolution protocol. CB2 activation can terminate inflammatory cascades that resist conventional intervention.
Biomarker blindness
Researchers enthuse about specialized pro-resolving mediators in picomolar ranges, apparently oblivious that endocannabinoids function identically and are more accessible to measurement. Simple biomarkers like red blood cell arachidonic acid could predict the activities of both pathways, but such integration requires thinking beyond departmental boundaries.
Translation failures explained
The notorious gulf between preclinical promise and clinical disappointment in anti-inflammatory therapeutics finally makes sense. When you’re manipulating half the network, comprehensive efficacy becomes impossible. Interventions addressing both axes might achieve the synergistic outcomes that single-pathway approaches consistently miss.
The Structural Pathology
This isn’t accidental, rather it is systematic and emblematic of a bigger issue:
- Eicosanoid researchers inhabit immunology journals.
- ECS investigators cluster in neuroscience publications.
- Grant structures reward narrow specialization over integration.
- Career advancement depends on tribal loyalty, not broad understanding.
- Regulatory stigma makes “cannabinoid” research institutionally riskier.
The result: intellectual apartheid masquerading as scientific rigor.
Time for intellectual honesty
Arachidonic acid doesn’t consult departmental affiliations before entering metabolic pathways, it becomes both prostaglandins and endocannabinoids simultaneously. Our research paradigms should reflect this biochemical reality.
Rather than “reprogramming inflammation” through eicosanoid tunnel vision, we need “substrate-conscious medicine” that recognizes interconnected signaling networks. The answer isn’t more sophisticated targeting of individual pathways, it’s understanding that substrate control affects multiple systems that must be considered holistically.
A call to action
To inflammation researchers: Your next arachidonic acid paper is incomplete without considering endocannabinoid implications.
To ECS researchers: Your substrate-driven findings have profound implications for inflammation that extend far beyond your traditional audience.
To funding agencies: Reward interdisciplinary collaboration over territorial protection.
To journal editors: Demand broader mechanistic thinking from your reviewers and authors.
The Kuipers-Declèves parallel universe phenomenon proves that even brilliant researchers can study identical mechanisms while remaining oblivious to each other’s work. The Kolawole 2025 review demonstrates that this blindness persists and may be getting worse.
The science demands better. Patients deserve better. It’s time to abandon the comfortable delusions of reductionist thinking.
What other critical connections are we missing due to academic tunnel vision? How many therapeutic opportunities remain unexplored because researchers refuse to look beyond their silos?
References:
Declèves AE, Mathew AV, Armando AM, et al. AMP-activated protein kinase activation ameliorates eicosanoid dysregulation in high-fat-induced kidney disease in mice. J Lipid Res. 2019;60(5):937-952. doi:10.1194/jlr.M088690
Kolawole OR, Kashfi K. Reprogramming inflammation: Mechanisms and therapeutic targeting of eicosanoids and pro-resolving mediators. Eur J Pharmacol. 2025;1003:177924. doi:10.1016/j.ejphar.2025.177924
Kuipers EN, Kantae V, Maarse BCE, et al. High Fat Diet Increases Circulating Endocannabinoids Accompanied by Increased Synthesis Enzymes in Adipose Tissue. Front Physiol. 2019;9:1913. Published 2019 Jan 10. doi:10.3389/fphys.2018.01913

Comment
Comments are closed.