In recent years, the medical community has witnessed a revolution in the treatment of obesity with the advent of GLP-1 receptor agonists like Ozempic and Wegovy. These drugs have shown remarkable efficacy in promoting weight loss and improving metabolic health [1]. However, as our understanding of the complex interplay between various physiological systems deepens, an intriguing connection has emerged – the relationship between GLP-1 receptor agonists and the endocannabinoid system (ECS).
The Endocannabinoid System: A Key Player in Metabolic Regulation
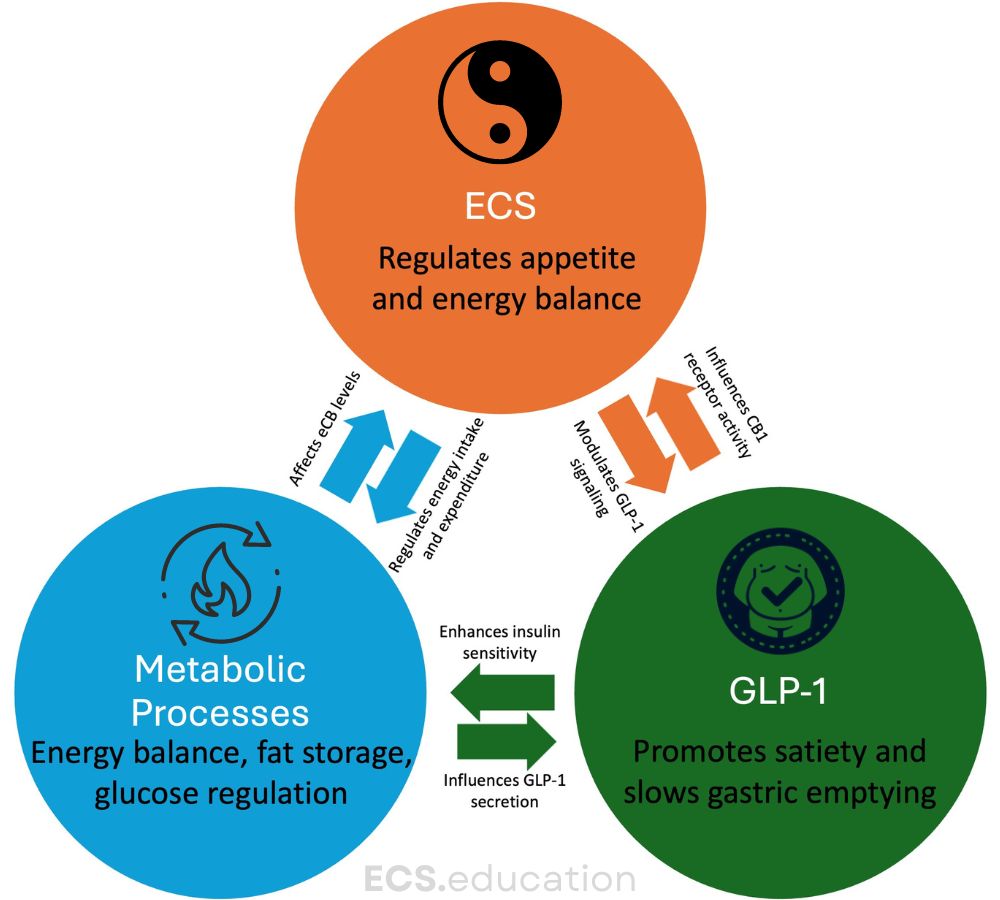
The ECS, a complex network of receptors, endogenous ligands, and enzymes, plays a crucial role in regulating energy balance, appetite, and metabolism [2]. This system, frequently overlooked in mainstream medicine (as evidenced by its absence in medical textbooks and lack of knowledge among healthcare professionals), is now recognized as a central player in maintaining homeostasis throughout the body.
Cannabinoid receptor type 1 (CB1) and type 2 (CB2) are the primary receptors of the ECS. CB1 receptors, in particular, are widely distributed in the brain and peripheral tissues, including those involved in metabolism such as the liver, adipose tissue, and pancreas [3]. This distribution pattern hints at the ECS’s potential influence on metabolic processes.
GLP-1 Receptor Agonists: More Than Meets the Eye
GLP-1 receptor agonists, initially developed for diabetes management, have emerged as potent anti-obesity medications. These drugs mimic the action of the incretin hormone GLP-1, promoting satiety, slowing gastric emptying, and improving insulin sensitivity [4].
But could there be more to their mechanism of action? Recent research suggests that the efficacy of GLP-1 receptor agonists might be partially mediated through interactions with the ECS/eCBome. A groundbreaking study by Matias et al. (2024) found that endocannabinoid-related molecules could predict the metabolic efficacy of GLP-1 receptor agonists in humans with obesity [5]. This finding opens up a new avenue for understanding and potentially enhancing the effects of these drugs.
Dietary Lipids and GLP-1 Secretion: A Natural Approach to Satiety
While discussing GLP-1 receptor agonists, it’s important to note that certain dietary lipids can naturally stimulate GLP-1 secretion, contributing to increased satiety. Research has shown that not all fats are equal when it comes to triggering GLP-1 release:
- Omega-3 fatty acids, particularly EPA and DHA, have been found to have a high effect on GLP-1 secretion, with strong evidence supporting this claim [6,7].
- Monounsaturated fatty acids (MUFAs), such as those found in olive oil, also strongly promote GLP-1 release, although the evidence is moderate [8,9].
- Omega-6 fatty acids, like linoleic acid, have a moderate to high effect on GLP-1 secretion, with moderate supporting evidence [6,7].
- Medium-chain triglycerides (MCTs) show a moderate effect on GLP-1 secretion, though the evidence is currently limited [10].
- Saturated fatty acids, such as those found in butter, have a low to moderate effect on GLP-1 secretion, with moderate evidence supporting this finding [9,11].
- Trans fatty acids appear to have a low effect on GLP-1 secretion, although the evidence for this is limited.
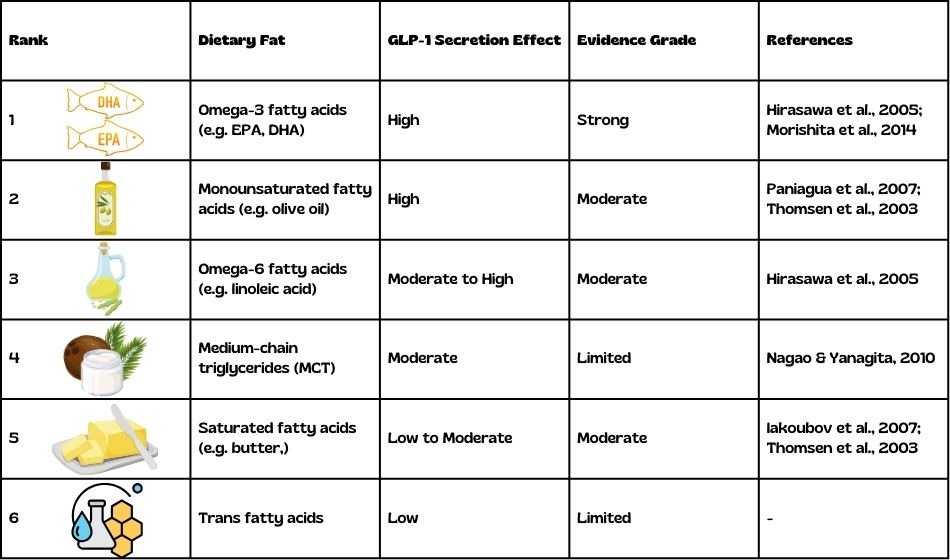
This differential effect of dietary lipids on GLP-1 secretion adds another layer of complexity to our understanding of metabolism and appetite regulation. It suggests that dietary composition, particularly the type of fats consumed, may play a role in naturally modulating GLP-1 levels and influencing satiety.
These findings could have implications for dietary recommendations in obesity management, potentially complementing pharmacological approaches using GLP-1 receptor agonists. For example, diets rich in omega-3 fatty acids and MUFAs might be beneficial in promoting GLP-1 secretion and improving metabolic health [12].
However, it’s important to note that while certain fats may stimulate GLP-1 secretion more than others, a balanced approach to fat consumption is crucial for overall health, considering other metabolic effects of different types of fats. The complex interplay between dietary fats, GLP-1 secretion, and metabolic health underscores the need for personalized nutritional strategies in managing obesity and related metabolic disorders.
The CB1-GLP-1 Connection: A Multifaceted Synergistic Relationship
Recent research has uncovered a fascinating interplay between the endocannabinoid system and the incretin system, suggesting that targeting both systems simultaneously could lead to more effective treatments for obesity and metabolic disorders.
A revealing study by Zizzari et al. (2021) demonstrated that CB1 and GLP-1 receptors engage in extensive cross-talk, providing new therapeutic avenues for obesity treatment [13]. This research revealed several key findings:
- Bidirectional modulation between GLP-1R and peripheral CB1R signaling in controlling energy homeostasis.
- Enhanced efficacy of GLP-1-mediated effects through CB1 receptor blockade.
- Partial dependence of CB1-induced weight loss on GLP-1R signaling.
- Synergistic effects of combined CB1R blockers and GLP-1R agonists in reducing body weight and fat mass.
- Improved metabolic parameters, including insulin resistance and nonalcoholic fatty liver disease, with combination therapy.
Oleoylethanolamide: A Bridge Between the ECS and GLP-1 Signaling
Adding another layer to this intricate relationship, a study by Brown et al. (2018) revealed that the hunger-suppressing effects of oleoylethanolamide (OEA) involves GLP-1 release [14]. This research brings yet another dimension to our understanding of the ECS-GLP-1 interaction:
- The study demonstrated that OEA-activation of GPR119 mobilises GLP-1 secretion.
- When combined with exendin-4 (a GLP-1 receptor agonist), OEA led to greater weight loss in obese mice compared to either compound alone.
- The OEA and exendin-4 combination promoted increased energy expenditure in diet-induced obese mice, suggesting multiple mechanisms contributing to weight loss.
- The research explored the effects of OEA and GLP-1 on various signaling pathways, including canonical GLP-1R signaling, AMPK, Akt, mTOR, and glycolysis.
- Interestingly, OEA enhanced canonical GLP-1R signaling when combined with GLP-1 but not with exendin-4, highlighting the complexity of these interactions and the need for further research.
Importantly, a study published in JAMA Psychiatry (2014) adds another crucial dimension to our understanding of OEA’s role in obesity [15]. This research revealed that OEA’s effects on food-related brain activation and body mass index (BMI) differ significantly between obese and non-obese individuals:
- In obese individuals, OEA levels showed a trend towards a positive correlation with BMI, while in non-obese individuals, this relationship was inverse.
- Non-obese individuals with higher OEA levels exhibited increased insular brain activity in response to food stimuli, whereas this relationship trended inversely for obese patients.
- The study suggested that the identified brain areas may be involved in suppressing food-liking reactions in non-obese individuals, potentially through OEA-mediated signaling.
These findings highlight the complex role of OEA in regulating food intake and reward processing, which appears to be altered in the context of obesity. This differential effect of OEA in obese versus non-obese individuals adds an important consideration for the development of combination therapies targeting both the endocannabinoid system and GLP-1 signaling.
The findings also suggest a big potential for future multimodal treatment protocols, leveraging the combined power of GLP-1 receptor activation, CB1 receptor modulation, and eCBome mediators like OEA, for increased therapeutic efficacy.
Rethinking Obesity Treatment: A Holistic Approach
The emerging connections between GLP-1 receptor agonists, the endocannabinoid system, and eCBome mediators like OEA underscore the need for a more holistic approach to obesity treatment. Rather than focusing on single pathways, future therapies might target multiple systems to achieve optimal results.
This approach aligns with the growing recognition of obesity as a complex, multifactorial disease influenced by genetic, environmental, and behavioral factors [16]. By addressing multiple aspects of energy balance and metabolism simultaneously, we may be able to develop more effective and personalized treatments.
Challenges and Future Directions
While the potential of combining GLP-1 receptor agonists with ECS modulation is exciting, it’s important to note that previous attempts to target the ECS for obesity treatment have faced challenges. The CB1 receptor antagonist rimonabant, once hailed as a promising anti-obesity drug, was withdrawn from the market due to psychiatric side effects [17]. However, the failure of rimonabant should not discourage further research in this area. Instead, it highlights the need for more nuanced approaches to ECS modulation. Future studies should focus on developing peripherally restricted CB1 modulators or exploring other components of the ECS as potential targets [18].
Conclusion: Future Multimodal Obesity Treatments?
The intersection of GLP-1 receptor agonists, the endocannabinoid system, and eCBome mediators like OEA represents a fascinating new frontier in obesity research. As we continue to unravel the complex interactions between these systems, we may unlock new possibilities for more effective and targeted obesity treatments.
This emerging field of research serves as a reminder of the importance of considering the body’s interconnected systems in developing therapeutic approaches. By embracing a more holistic view of metabolism and energy balance, we may be able to address the obesity epidemic more effectively than ever before.
As we move forward, it’s crucial that we continue to invest in research exploring these connections. The potential benefits extend beyond obesity treatment, potentially impacting our understanding and management of various metabolic disorders.
In the end, the story of GLP-1 receptor agonists, the endocannabinoid system, and eCBome mediators is not just about finding new drug targets. It’s about gaining a deeper understanding of the intricate systems that regulate our bodies and using that knowledge to improve human health. As we stand on the brink of this new frontier, the future of obesity treatment looks more promising than ever.
References:
- Liu Y, Ruan B, Jiang H, et al. The Weight-loss Effect of GLP-1RAs Glucagon-Like Peptide-1 Receptor Agonists in Non-diabetic Individuals with Overweight or Obesity: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. Obesity Reviews. 2023;24(9):e13623. doi:10.1111/obr.13623
- Di Marzo V, Silvestri C. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013;17(4):475-490. doi:10.1016/j.cmet.2013.03.001
- Murphy T, Le Foll B. Targeting the Endocannabinoid CB1 Receptor to Treat Body Weight Disorders: A Preclinical and Clinical Review of the Therapeutic Potential of Past and Present CB1 Drugs. Biomolecules. 2020;10(6):855. Published 2020 Jun 4. doi:10.3390/biom10060855
- Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
- Matias I, Lehmann EW, Zizzari P, et al. Endocannabinoid-related molecules predict the metabolic efficacy of GLP-1 receptor agonism in humans with obesity. J Endocrinol Invest. 2024;47(5):1289-1294. doi:10.1007/s40618-023-02228-8
- Hirasawa A, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11(1):90-94.
- Morishita M, et al. Enteral insulin induces GLP-1 secretion through TRPA1 and TRPV1-mediated calcium influx in enteroendocrine L cells. Endocrinology. 2014;155(11):4356-4369.
- Paniagua JA, et al. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care. 2007;30(7):1717-1723.
- Thomsen C, et al. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr. 1999;69(6):1135-1143.
- Nagao K, Yanagita T. Medium-chain fatty acids: functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol Res. 2010;61(3):208-212.
- Iakoubov R, et al. Essential role for protein kinase Cζ in oleic acid-induced glucagon-like peptide-1 secretion in vivo in the rat. Endocrinology. 2007;148(3):1089-1095.
- Rocca AS, et al. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. 2001;142(3):1148-1155.
- Zizzari P, He R, Falk S, et al. CB1 and GLP-1 Receptors Cross Talk Provides New Therapies for Obesity. Diabetes. 2021;70(2):415-422. doi:10.2337/db20-0162
- Brown JD, McAnally D, Ayala JE, et al. Oleoylethanolamide modulates glucagon-like peptide-1 receptor agonist signaling and enhances exendin-4-mediated weight loss in obese mice. Am J Physiol Regul Integr Comp Physiol. 2018;315(4):R595-R608. doi:10.1152/ajpregu.00459.2017
- Grosshans M, Schwarz E, Bumb JM, et al. Oleoylethanolamide and Human Neural Responses to Food Stimuli in Obesity. JAMA Psychiatry. 2014;71(11):1254–1261. doi:10.1001/jamapsychiatry.2014.1215]
- Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33(7):673-689.
- Sam AH, Salem V, Ghatei MA. Rimonabant: From RIO to Ban. J Obes. 2011;2011:432607. doi:10.1155/2011/432607
- Tam J, Vemuri VK, Liu J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120(8):2953-2966. doi:10.1172/JCI42551

Comments (2)
Comments are closed.